

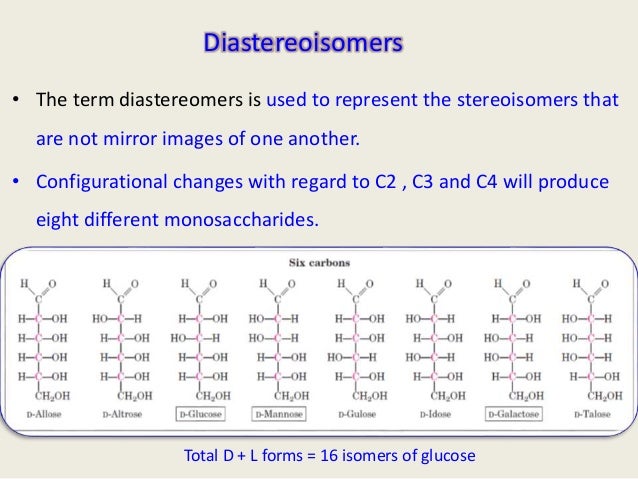
So, for a molecule like glucose, you have 4 chiral carbons. However, as you go down the line to more complex carbohydrates, you get more and more stereocenters. The simplest one, glyceraldehyde, only has one. Stereochemistry of CarbohydratesĬarbohydrates have multiple stereocenters. Then, say how many carbons you have in the molecule and add -ose to signify the carbohydrate.
The nomenclature of ketoses follows the same principles as for aldoses: you start by saying keto- to point out the functional group. Most common ketoses have a ketone functional group on the second carbon in the chain. As ketoses contain a ketone functional group, we obviously cannot have it at the beginning of the chain. And we finish by adding the -ose ending to specify that it’s a carbohydrate we’re dealing with. We then say how many carbons are there in the molecule. As you can guess, aldo- goes together with an aldehyde, and keto- with the ketone-containing carbohydrates.Īs you can see from these examples, we start the name by saying that the molecule is an aldehyde using the aldo- prefix. We specify this in the name by adding aldo– or keto– prefix to the carbohydrate name. However, sugars will only have one aldehyde OR one ketone functional group. Carbohydrates generally have multiple alcohol functional groups, so we never focus on those. Sugars, or carbohydrates, have two major functional groups: an aldehyde or a ketone (both are collectively called carbonyls), and an alcohol functional group. Naming the Major Functional Group in a Carbohydrate So, when I say that we’re dealing with a hexose, that doesn’t mean much except for the fact that the molecule contains 6 carbons. This list includes glucose, galactose, fructose, mannose, etc. For instance, there are 24 different hexoses (12 of which exist in nature). This type of a name, however, doesn’t tell us the exact nature of the molecule. For instance, the glucose is an example of a hexose because it has six carbons in the molecule. For instance, a triose is a carbohydrate with 3 carbons, while hexose is a carbohydrate with 6 carbons in the molecule. We use the greek numerals to call the number, aka tri-, tetra-, penta-, hexa-, and add the ending -ose to denote that it’s a carbohydrate. It can be a spontaneous one, which is a slow process, or can be catalyzed by the enzymes.The simplest carbohydrate has 3 carbons.
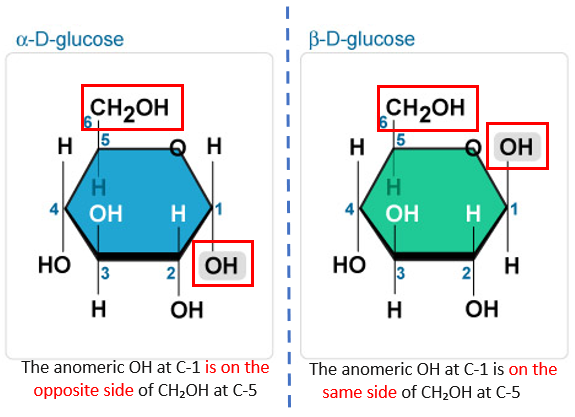
The -OH group plays a wide role in the formation of epimers and anomers too.Some examples of Epimers are anomeric forms of sugars and other sugars except not anomers (such as D-glucose and D-galactose).Furthermore, they differ in their physical and chemical properties. Epimers are not mirrored images of each other. All epimers are diastereomers but all diastereomers are not necessarily epimers.For alpha, the -OH is on the right but for beta, the -OH is on the left. The configuration at one chiral centre is different in both the anomers. In the above figure, for the alpha anomer of glucose, the -OH group points down, which is attached to the anomeric carbon (C1), and for the beta anomer of glucose, the -OH points up, which is attached to the anomeric carbon.

We can understand this term with an example: Anomers also have names to differentiate their forms- the alpha designation is given to the form where the OH group points down on the anomeric carbon, and the beta designation is given to the form where the OH group points up on the anomeric carbon. The anomeric carbon refers to the carbon atom in the cyclical carbon chain, reduced to form a ketone. They differ in 3-D structure from each other only at one site, that is, the hemiacetal/ hemiketal carbon, which is also called the anomeric carbon. Their physical, as well as chemical properties, are different from each other.Ī particular type of Epimers is found in carbohydrate (sugar) molecules which are known as Anomer.They do not have mirror images of each other.They are different from each other in a few chiral carbons, but not all chiral carbons.Epimers differ from each other in one chiral carbon in the configuration but can contain more than one chiral carbon.The main characteristics of epimers are as follows: Read More: Difference Between Enantiomers & Diastereomers Likewise, D-glucose and D- galactose are the most suitable examples of Epimers.


 0 kommentar(er)
0 kommentar(er)
